UDI Management in meddevo
Basic UDI-DI - General Information
The Basic UDI-DI is a unique code that identifies a product model or product family and serves as the primary key for records in the European EUDAMED database. It is not affixed directly to the product or its packaging, but is purely an administrative and database identifier required for administrative and regulatory purposes. The Basic UDI-DI is independent of individual packaging units and is required to group multiple UDIs (device identifiers) together.
What the Basic UDI-DI does:
Grouping of products:
It links medical devices with the same intended use, risk class, and similar design and manufacturing characteristics.
Primary key in EUDAMED:
It is the primary key for identifying product groups in the EUDAMED database.
Linking documents:
It can be found in relevant documents such as declarations of conformity and technical documentation.
What the Basic UDI-DI is not:
- Not on products: It is not affixed to the product itself or its packaging.
- Not a product or packaging identifier: It is not an identifier for a specific unit, but for a group of devices.
Difference from the UDI-DI:
- The Basic UDI-DI identifies an entire product family or product model.
- The UDI-DI identifies each individual product within that family and is affixed to the product or packaging.
Basic UDI-DI Codes and relevant information in meddevo
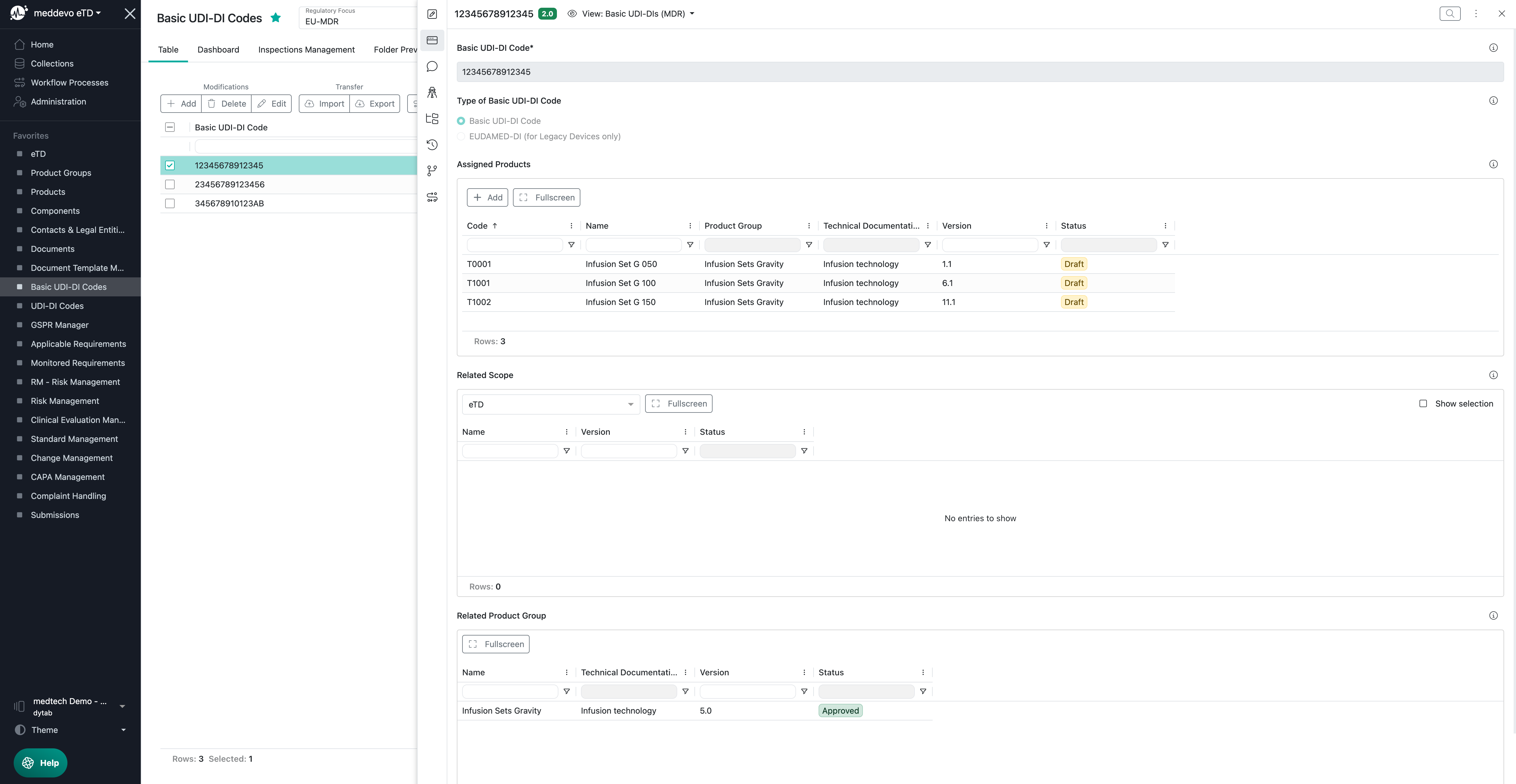
The Basic UDI-DI Codes collection is the place to manage your codes:
Of course you can import all your Basic UDI-DI codes via an Excel import.
All Basic UDI-DI code relevant regulatory information will be managed in your Products collection:
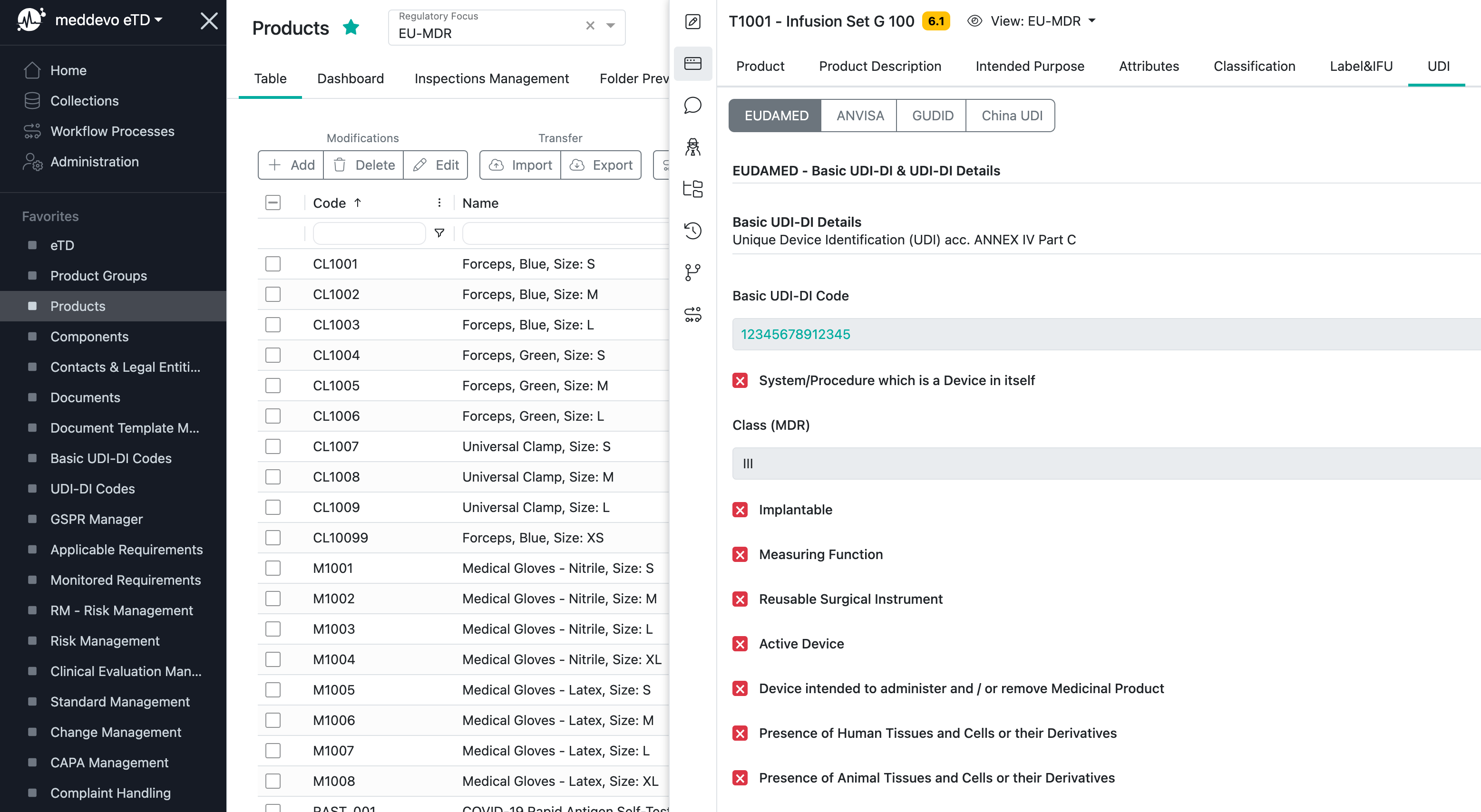
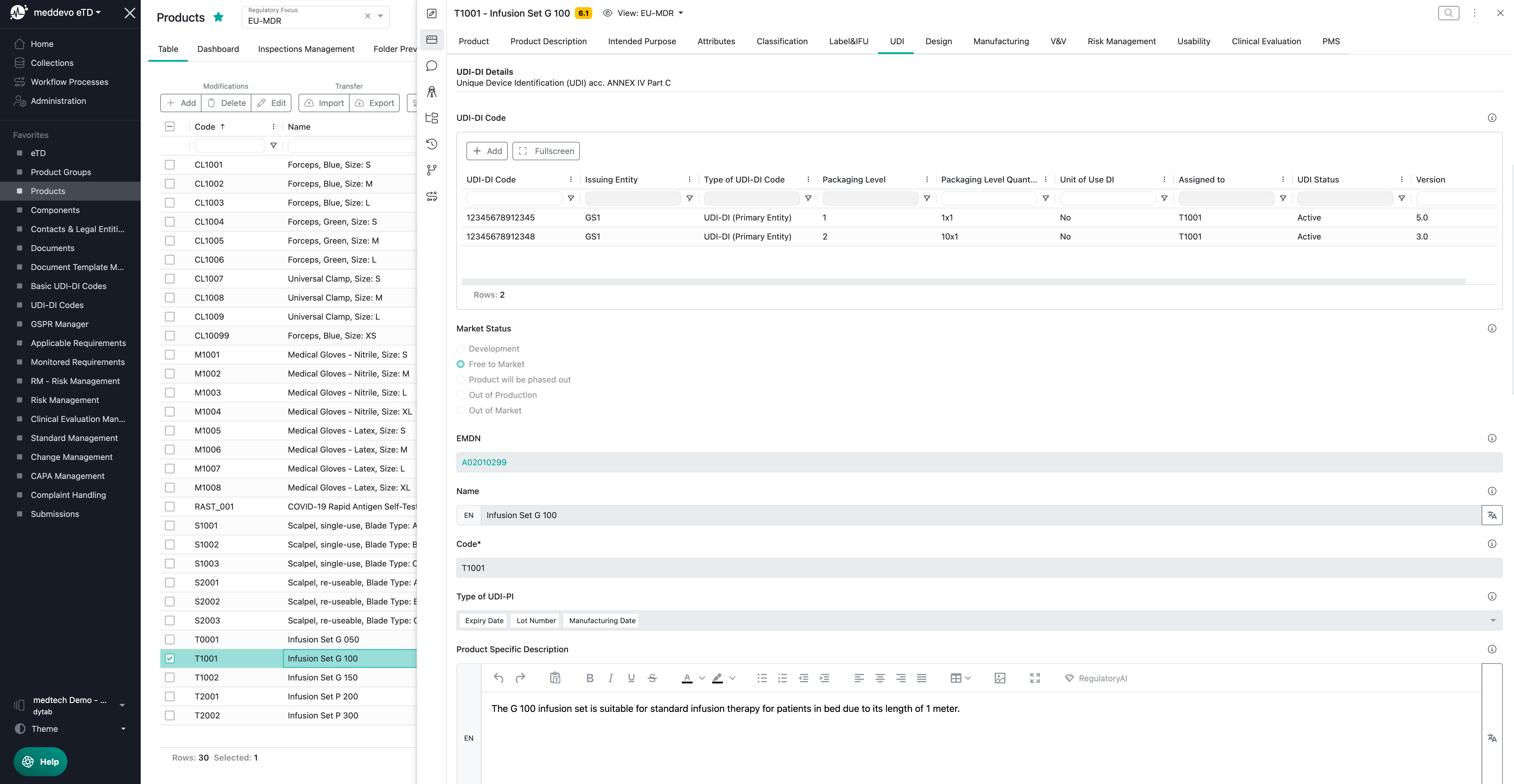
UDI-DI Codes and relevant information in meddevo
The UDI-DI Code Collection is where you can manage your codes and EUDAMED-relevant attributes:
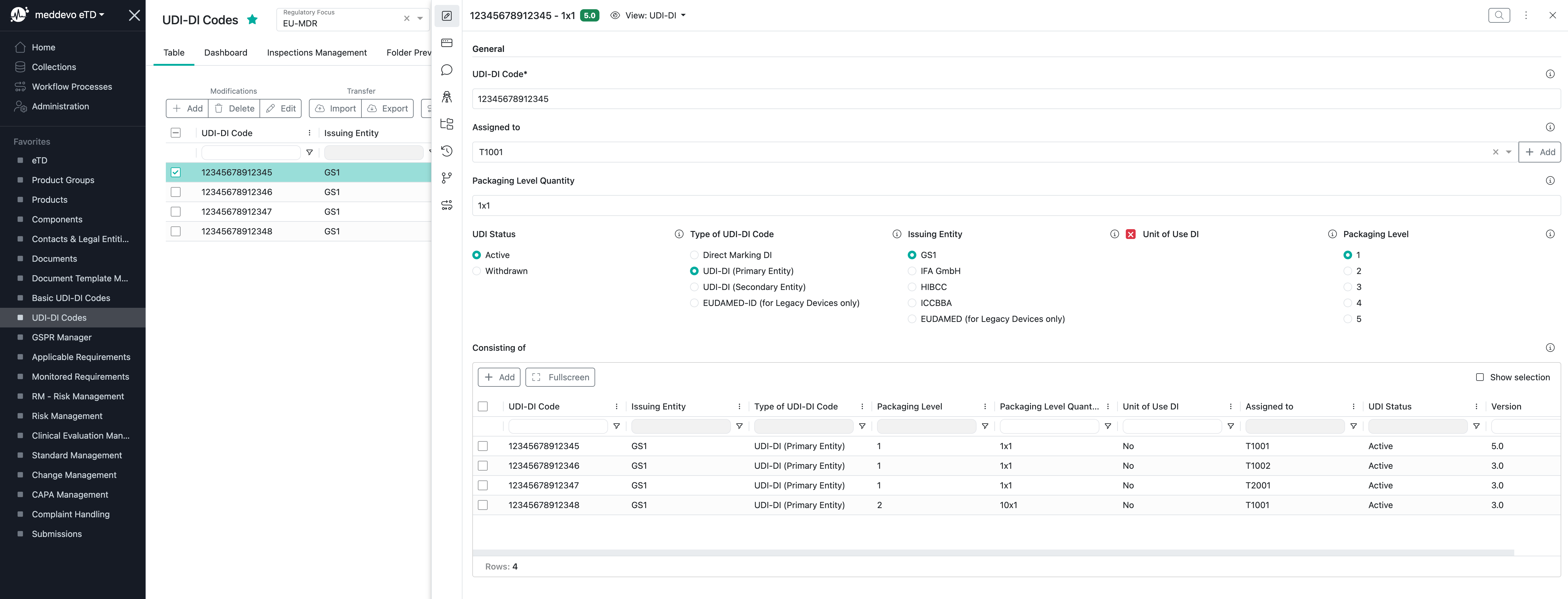
Of course you can import all your UDI-DI codes via an Excel import.
Description and explanation of the UDI-DI relevant fields:
- "UDI-DI Code" - here you can place your UDI-DI code.
- "Assigned to" - here you can assign the product linked to the UDI-DI code
- "Packaging Level Quantity" - here you can define the quantity of your sales unit, e.g. 1x1, 10x1, 30x5 etc.
- "UDI Status" - here you can define whether the UDI-DI code was withdrawn or if it is still active.
- "Type of UDI-DI Code" - here you are able to differ between:
- Direct Marking DI - if you have a reusable device, which requires a direct marking
- UDI-DI (Primary Entity) - if you use your standard UDI-DI codes offered by your first Issuing Entity
- UDI-DI (Secondary Entity) - if you have two Issuing Entities in parallel
- EUDAMED-ID (for Legacy Devices only) - if you need to register your legacy devices
- "Issuing Entity" - here you can choose one of the five official UDI issuing entities
- "Unit of Use DI" - this is only required, if your smallest sales unit contains more than one product. In this case you need to define a UoU DI which is not physically available on your product. This is only required for EUDAMED and the complaints from customers.
- "Packaging Level" - here you define the different packaging levels of your official sales units. Shipping boxes and containers are not considered.
- For example:
- Primary Packaging (Level 1) with contents 1x1 (defined under packaging quantity)
- Secondary Packaging (Level 2) with contents 5x1
- Tertiary Packaging (Level 3) with contents 30x5.
- Pallets and all logistical packaging levels are not relevant.
- For example:
- "Consisting of" - if you define a UDI-DI for a higher packaging level, here you are able to choose the respective UDI-DI codes of the lower levels of the same product.
All UDI-DI code relevant regulatory information required for the EUDAMED registration will be managed in your Products collection and summarized in the "UDI" section: