Clinical Evaluation Management - Overview
Clinical Evaluation Management
- Click on Collections.
- Open the collection "eCER - Clinical Evaluation Management".
In the Project Data section you can manage all project relevant information:
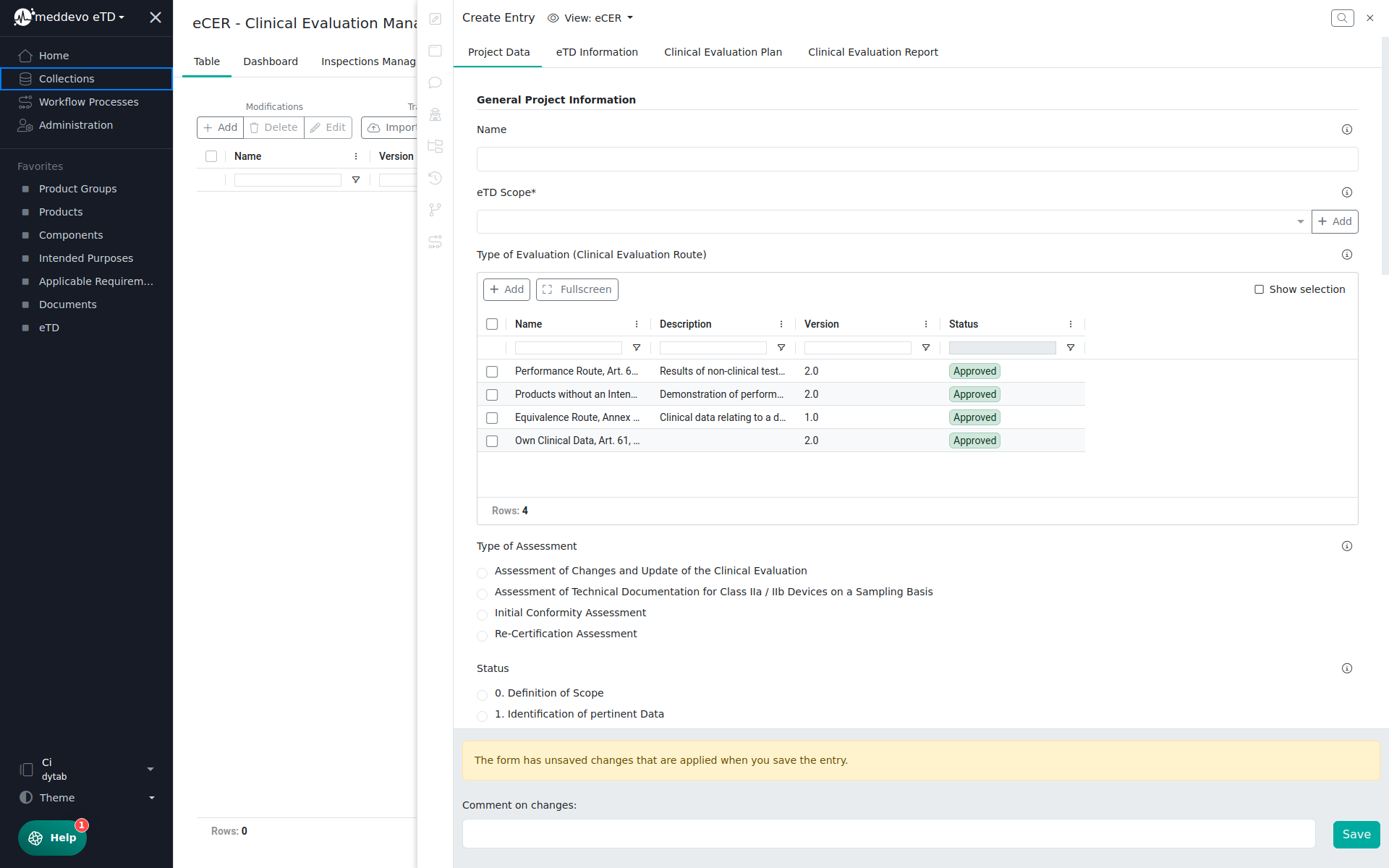
If you create the new clinical evaluation project please fill in at least the Name and set the eTD Scope (for which technical documentation you want to create the CEP and CER)
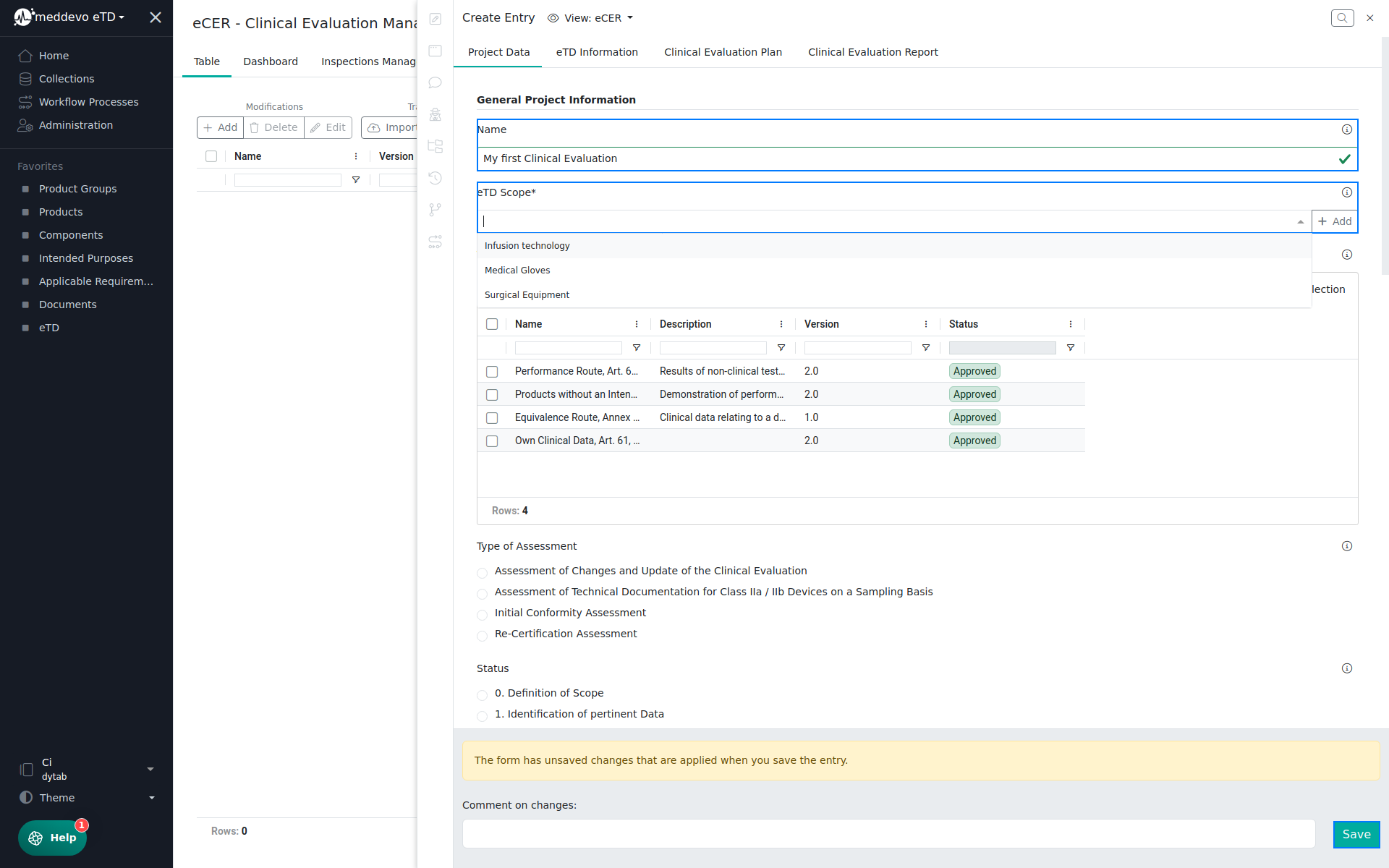
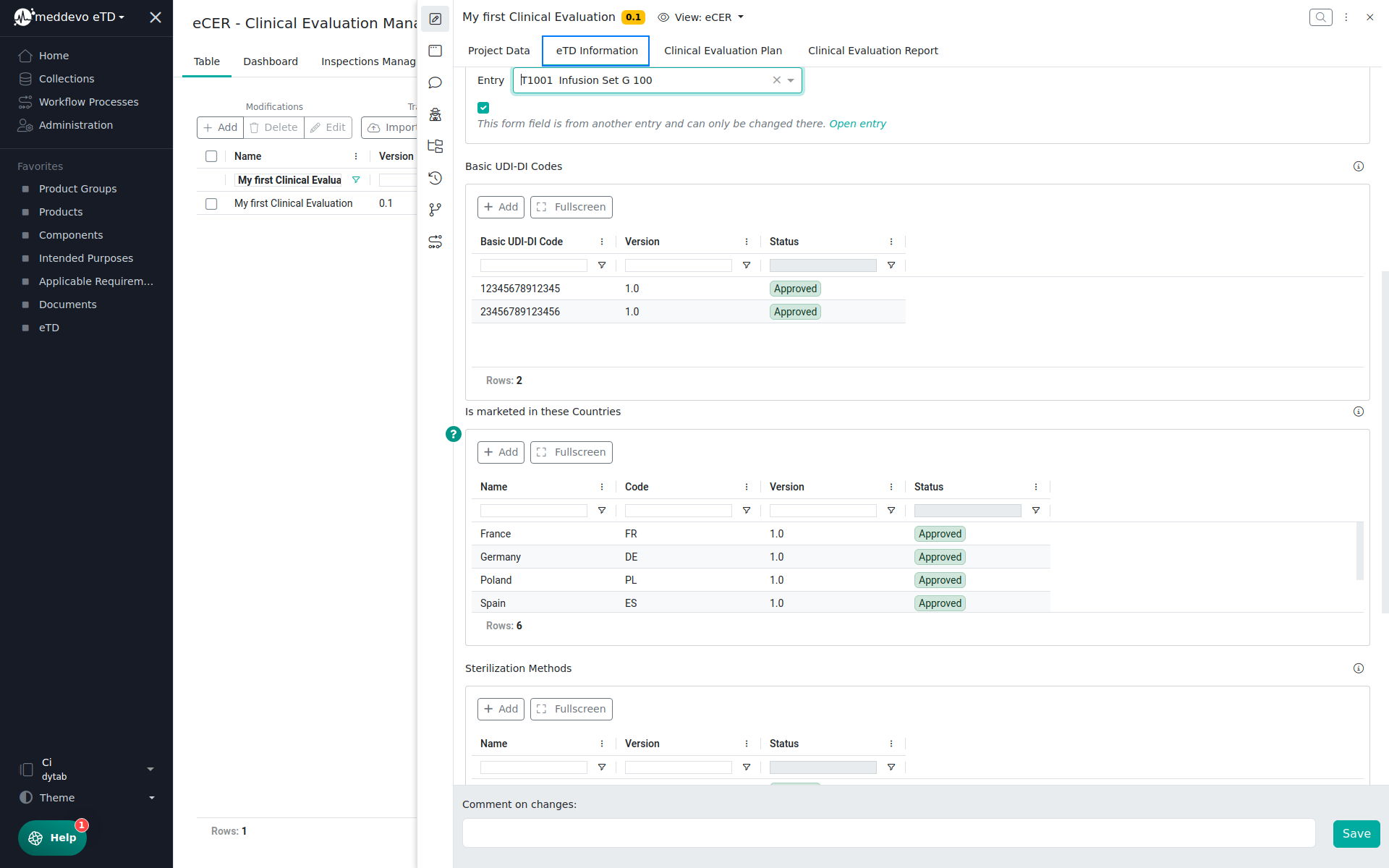
In the eTD Information section you can review all eTD information, which are automatically pulled from the referenced collections (eTD, Product Groups and Products), once you have set the eTD Scope and saved your project:
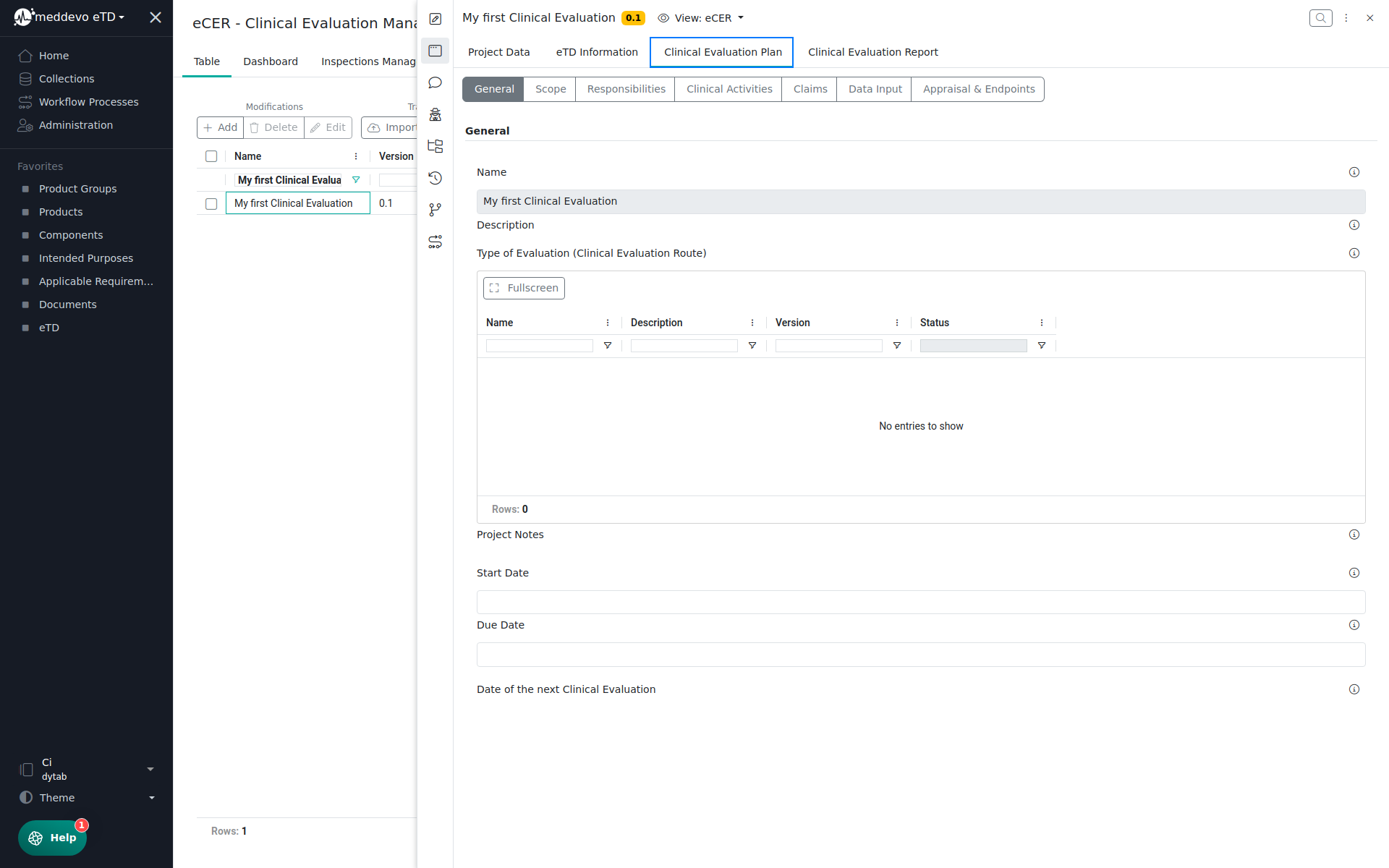
In the Clinical Evaluation Plan section you can manage all CEP relevant information:
Obviously in the Clinical Evaluation Report section you can manage all CER relevant information:
Once you are ready and all fields are filled, you can start the approval workflow, that all people involved in the project are able to perform the review and the approval of the CEP/CER relevant data. If the project is approved, you can create the CEP/CER based on our pre-defined meddevo Writer templates:
https://manual.dytab.net/en/article/creation-of-cep-cer-based-on-a-writer-template
