GSPR (General Safety and Performance Requirements) in meddevo
What is GSPR?
GSPR stands for General Safety and Performance Requirements. These requirements are a key part of the EU Medical Device Regulation (MDR) and In-Vitro Diagnostic Regulation (IVDR). They ensure that medical devices are safe and perform as intended. Every manufacturer must demonstrate compliance with the GSPR for all relevant products before placing them on the market.
Where can I find and use GSPR in meddevo?
You can find the GSPR Manager in your dashboard, under the Submission & Audits section.
1. Select the GSPR Evaluation Project you want to work on or create a new one by clicking "+ Add".
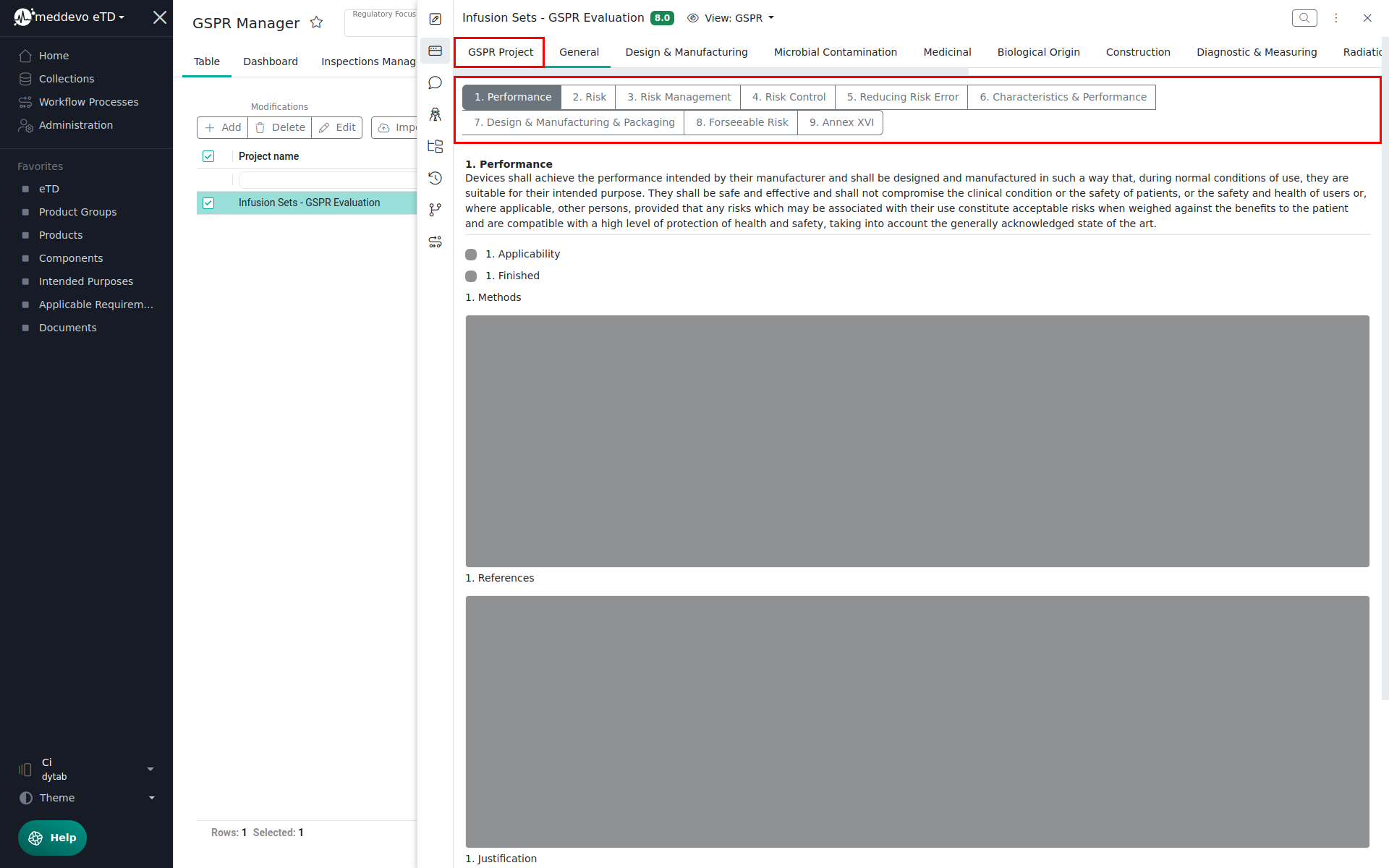
The GSPR View will open, with several tabs:
- Project General Information: Here you see general details such as the list of products included in the evaluation.
- GSPR Structure: The other tabs follow the same structure as in the EU MDR, for example:
- General Requirements (with further subtabs, e.g. 1. Performance, 2. Risk, 3. Risk Management, etc.)
- Each requirement contains:
- Description
- Applicability (checkbox)
- Finished (checkbox)
- Methods (link to applicable requirements)
- References (links to related collections)
- Justification (free text field)
On the left side of the GSPR View, you will find an edit symbol to change entries or use other action buttons, which are consistent across meddevo views.
How to create a GSPR Document via Template
Once you have completed the data entry in the GSPR Manager, you can easily generate a GSPR document using the provided meddevo template:
- Click on Create Document using the GSPR template called "General Safety & Performance Requirements (GSPR) DE/EN".
- The tool collects all entered data and generates a compliant GSPR document acc. MDCG 2021-08 with just a few clicks.
For more information on creating writer documents, see the detailed guide:
How to create a document with the meddevo Writer
Tip: Properly maintain your GSPR data and references. That way, your document will always be consistent with your product and audit trail in meddevo.
