Applicable Requirements/Regulatory Requirements - Overview (Standard Management 2.0)
Our new standard management consists of two parts: The Regulatory Requirements and the Applicable Requirements Collections.
In the past, this was only one collection - why two now? We decided to make them separate, with the advantage to provide the service of monitored standards. In the Regulatory Requirements meddevo will provide most common Standards and MDCG Documents. We will take care about up-to-dateness and validity of those entries.
All requirements that you specifically want to use for your portfolio are managed in the Applicable Requirements Collection, regardless of which area they come from. By linking your Applicable Requirements to the Regulatory Requirements you benefit from our standard monitoring while keeping full autonomy over your own relevant standards.
Please be aware, that all standards added to this collection by you need to be maintained by you. If you want meddevo to add a standard and track it for you, please get in contact with our customer service.
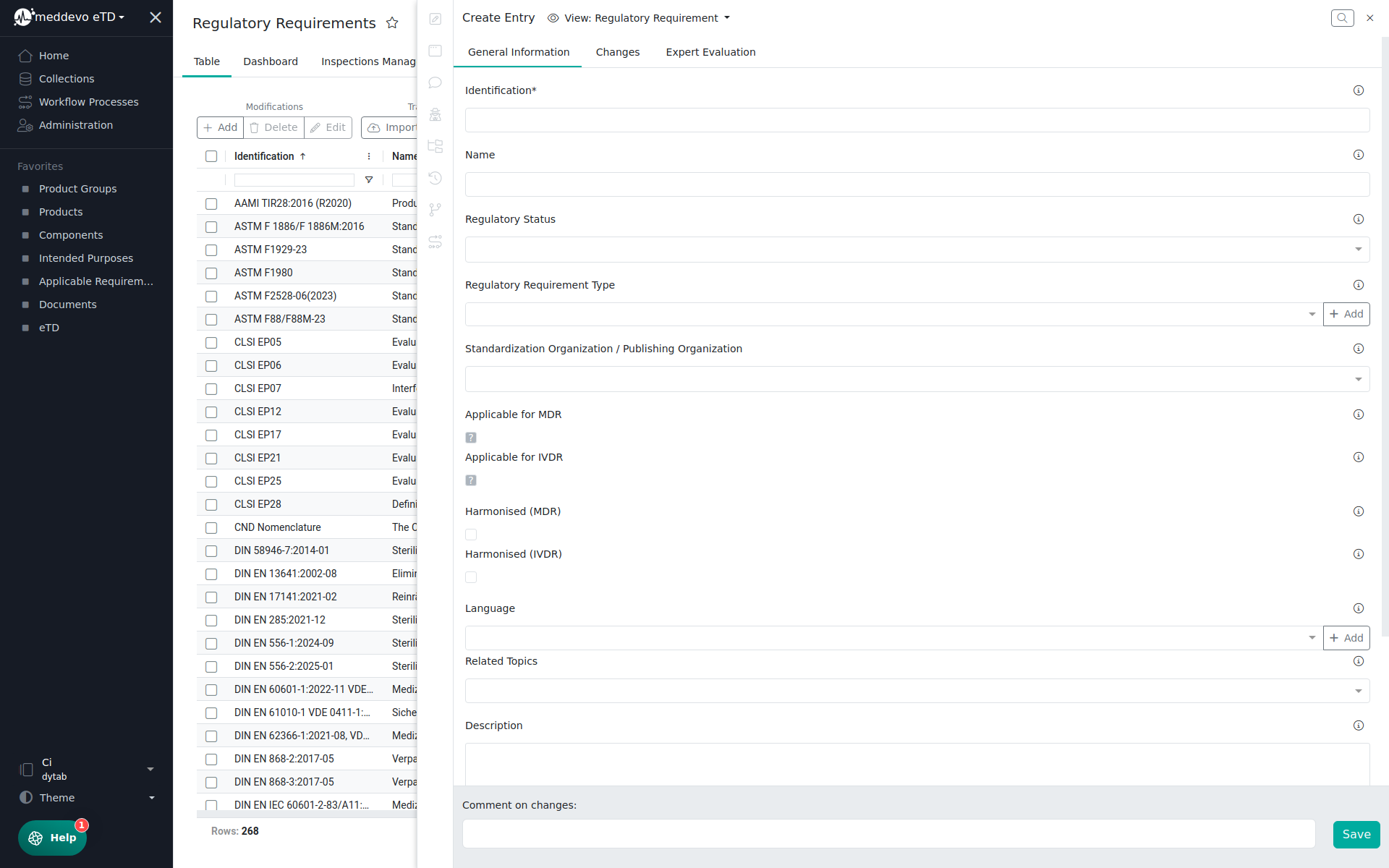
In the General Information Section you will find the following information:
- Identification
- Name
- Regulatory Status (Active, Draft, Withdrawn)
- Regulatory Requirement Type (Guidance, Legislation, Standard)
- Standardization Organization / Publishing Organization (CEN, CENELEC, DIN, EN, ETSI, IEC, ISO)
- Applicable & Harmonized for MDR/IVDR
- Language
- Related Topics (Biological Evaluation, Classification, Clinical, Cybersecurity, EUDAMED, Implantable Devices, Instruction for Use, Labelling, Legacy Devices, Notified Body, Others, Performance Evaluation, Post-Market-Surveillance, Quality Management, Risk Management, SARS-CoV-2/COVID-19, Software, Sterilisation, Unique Device Identification System, Usability Engineering, Vigilance)
In the Changes Section you will find information about any changes that took place between this standard and previous versions of it.
In the Expert Evaluation Section you can expect the conclusion from an MedTech expert:
- Expert Evaluation - coming soon
Again please be aware, that we can only offer expert evaluation for standards that are maintained by meddevo, not ones you add.
From this pool of information, you can than pick the standards relevant for you - with the Applicable Requirements Collection. And this is now the collection in which you can become active yourself!
To manage standards, you will need to assign the Requirement Type: Regulatory
Other types available, that will become important in future features of meddevo are:
- Design
- Marketing
- Production
- Risk related
- Usability
- User Requirement (URS)
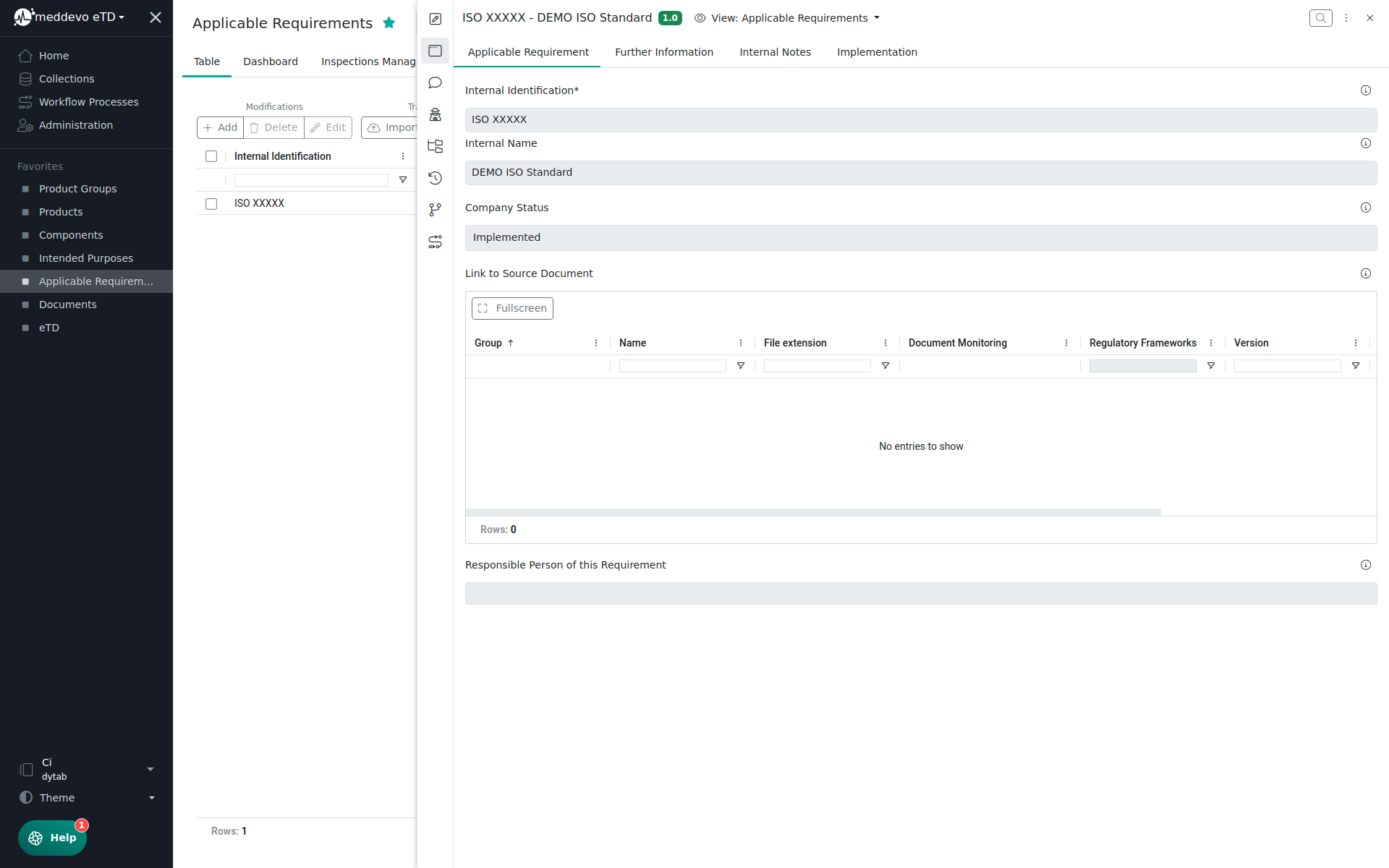
Besides that you can manage the following information in the Applicable Requirement and Further Information Section:
- Internal Identification
- Internal Name
- Regulatory Requirement Source
- Link to Source Document
- Responsible Person of this Requirement
- Type (Main/Content)
- Company Status (Implemented, Inactive, Open)
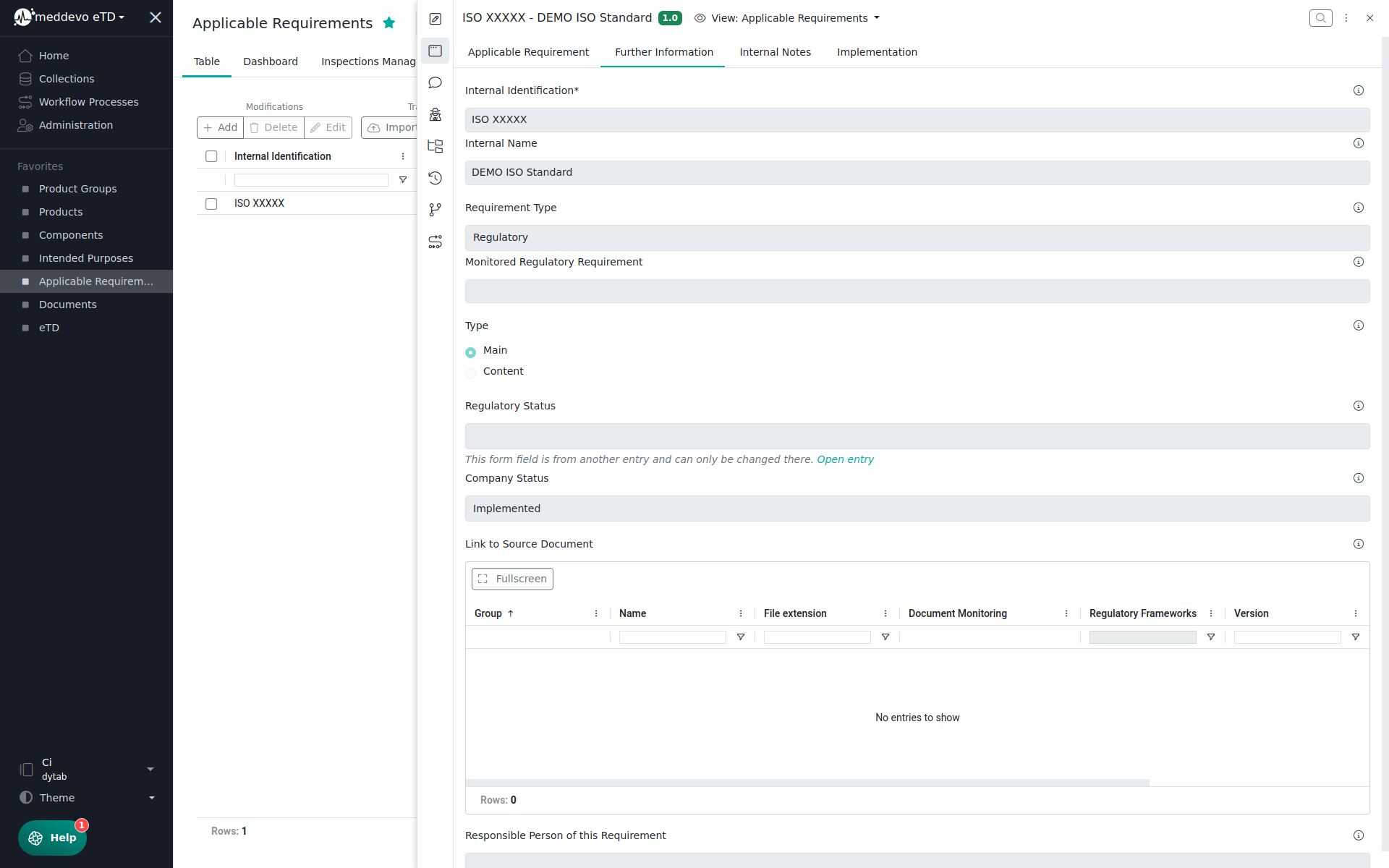
The Regulatory Status (managed in the Regulatory Requirements Collection) is displayed automatically here.
In the Internal Notes Section you are able to leave some Internal Notes (Summary Field) about the requirement.
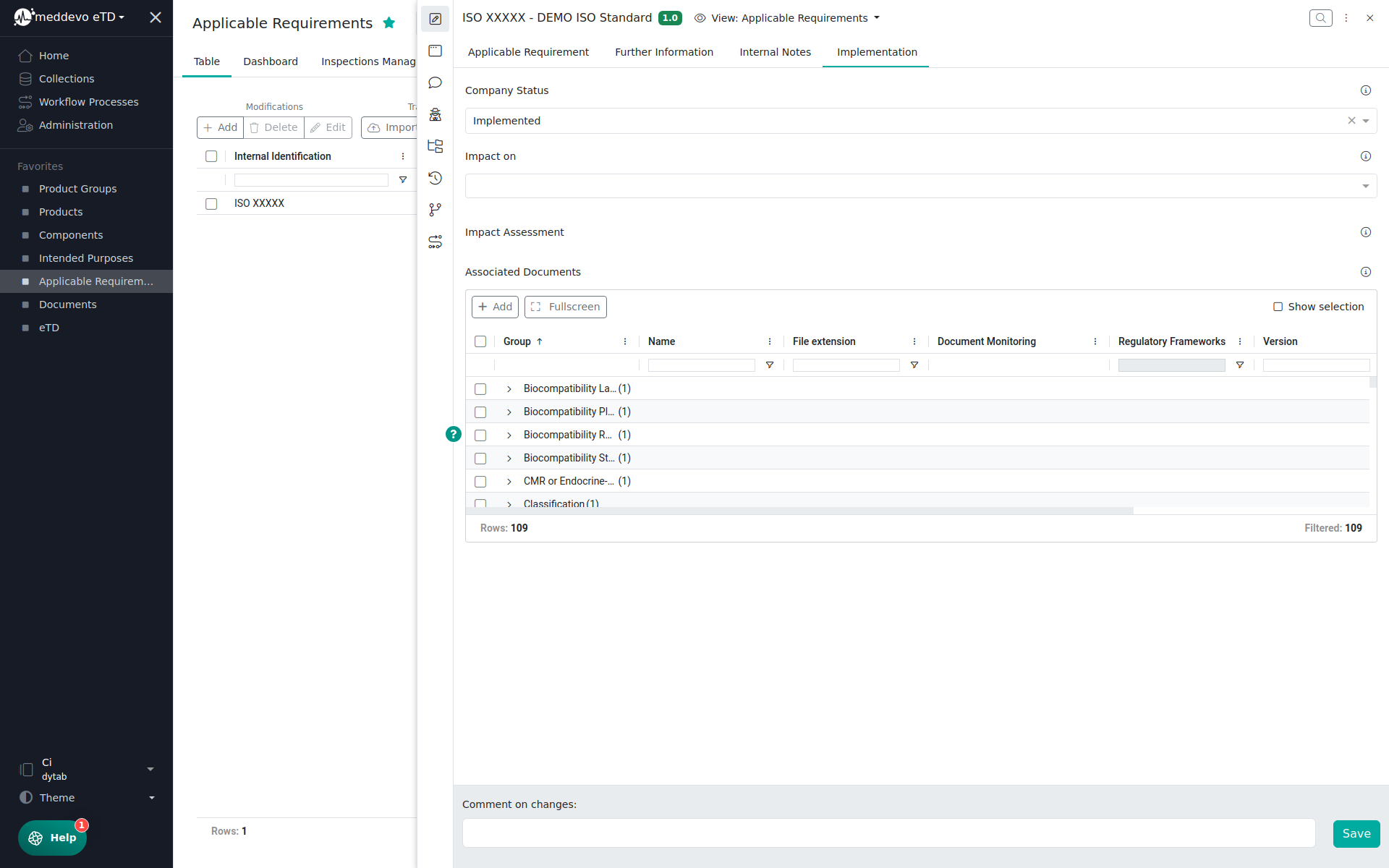
In the Implementation Section you can manage information related to the implementation of the requirement:
- Impact on (No Impact, Other, Processes, Products, Registrations, Risk Management)
- Impact Assessment (Summary Field)
- Associated Documents